
Image credit: Dr_Microbe/iStock/Getty Images Plus via Getty Images
Medically Important Foodborne Parasites: A Consequential Challenge for Food Safety Assurance
Foodborne parasitic diseases are often overlooked in food safety control schemes, even though they are known to pose a severe threat to human health
Of the numerous foodborne biological agents that threaten public health, foodborne parasites, it would appear, are a lower order of concern than are bacteria, viruses, and fungi. In some communities and for certain classes of food products, this risk assessment may indeed be a risky one. The list is long, and the illnesses and deaths caused by foodborne parasites are very consequential. More often than not, the reporting on biological hazards in human food are focused on bacterial pathogens. Almost on a daily basis, we are inundated with reports of products recalled due to Listeria contamination. By comparison, contemporary food safety literature on the subject of foodborne parasites is nearly mute.
Parasites are reported in all manner of foodstuffs and across the entire spectrum of the food supply. They infect fish and shellfish, fresh fruit, and vegetables, and they abound in the flesh and internal organs of the animals that are most frequently used as sources of meat. These non-bacterial agents of foodborne disease include helminths, ameba, sporozoa, and assorted other of the protozoa. They are complex organisms, and they are far more challenging than are bacteria when it comes to detection and control. For the purposes of risk assessment, food safety personnel must be cognizant of both the occurrence and significance of these medically important foodborne organisms.
When I first started working in the field of food microbiology (my education and prior work experience had been in medical microbiology), I had the occasion to ask the lab director about parasites and viruses and why they were not emphasized as agents of foodborne disease. He responded, "Stay focused on the bacteria." The challenge then, as it is now, had to do with the availability of reliable methods of detection. Most food testing labs, to this day, do not have good capability for detecting these non-bacterial disease-causing agents. In most instances, the methods of detection, qualification, and quantification for parasites are based on microscopy and a skilled parasitologist. Most food testing labs do not have a parasitologist on their staff. Moreover, most food microbiologists have not studied parasitology.
I recall vividly, as a young soldier, preparing a blood smear of blood obtained from an ill rifleman and finding it positive for malaria. Yes, I had seen the photos of the parasite in the textbooks and in my examination of previously prepared microscopic slides during training—but there was nothing so morbidly exhilarating as the firsthand experience of viewing, on a slide that I had prepared, this protozoan, fully engulfed and contained within a red corpuscle. I had a similar experience the first time I found a helminth oocyst in human excreta. Even then, we had to send the specimens to more sophisticated labs in order to confirm a definitive differential diagnosis. We knew the source of the malaria parasite, of course; but we were left with myriad unanswered questions about the source of the oocyst in the stool specimen. There were many plausible modes of transmission, and contaminated food or water were high on our risk ranking.
Food is a challenging material to examine for the presences of parasites. Looking for parasites on or in fresh fruit and vegetables, or seeking them out in the flesh of fish or other animal tissues, is very tedious and not necessarily standardized to the extent as are methods for the examination of other human-derived materials (e.g., sputum, urine, blood, or feces).1 Foodborne pathogenic agents comprise a diverse assortment of life forms including: viruses, fungi, algae, protozoa, and bacteria. The U.S., a country with well-developed public health infrastructure, an abundance of regulations, and regulatory oversight of its food system, reports 48 million case of foodborne illness and about 3,000 deaths annually that are attributed to the consumption of contaminated food. The organisms most frequently implicated as the etiological agents in these outbreaks are Salmonella spp., Campylobacter, Listeria, and Escherichia coli O157:H7.2 Other disease-causing agents, including foodborne parasites, also contribute significantly to this public health burden.
The U.S. Centers for Disease Control and Prevention (CDC) estimates that 31 of the most important known agents of foodborne disease found in foods consumed in the U.S. each year cause 9.4 million illnesses, 55,961 hospitalizations, and 1,351 deaths.2 CDC also reports that, "…not all agents of foodborne disease are known or can be counted as a 'known agent of foodborne disease.'" These other agents, which the agency call "unspecified agents," include:
- Known agents with insufficient data to estimate the agent-specific public health burden
- Known agents not yet recognized as causing foodborne illness (emerging pathogens)
- Microbes, chemicals, or other substances known to be in food with an unproven ability to cause illness
- Agents not yet described.
CDC reports suggest that these unspecified agents, in food consumed in the U.S., cause an additional 38.4 million gastroenteritis illnesses, 71,878 hospitalizations, and 1,686 deaths each year. After combining the estimates for the major known pathogens and the unspecified agents, the overall annual estimate of the total burden of disease due to contaminated food is 47.8 million illnesses, 127,839 hospitalizations, and 3,037 deaths.2
From these data it is clear that the unspecified agents play a big role in food safety. They are responsible for causing about 80 percent of foodborne illnesses annually and more than 50 percent of mortalities in the U.S. resulting from foodborne disease.
The top five pathogens (in the U.S.) contributing to acquired foodborne illnesses, in descending order of magnitude, are: norovirus, Salmonella (non-typhoidal), Clostridium perfringens, Campylobacter spp., and Staphylococcus aureus. However, when it comes to deaths caused by foodborne illness, the top five pathogens responsible are: Salmonella (non-typhoidal), Toxoplasma gondii, Listeria monocytogenes, norovirus, and Campylobacter spp.2,3 A World Health Organization (WHO) report from 2015 confirms that the parasitic worm, Taenia solium, is among the major causes of foodborne disease deaths globally.3
Parasites, as seen in the preceding data, are important foodborne pathogens. Their complex lifecycles, varied transmission routes, and prolonged periods between infection and manifestation of symptoms mean that the public health burden and relative importance of different transmission routes are often difficult to assess. Furthermore, there are challenges in detection and diagnostics.1 A Europe‐focused ranking exercise, using multicriteria decision analysis, identified potentially foodborne parasites of importance that are currently not routinely controlled in food. These are Cryptosporidium spp., Toxoplasma gondii, and Echinococcus spp.4 The differences between these three parasites are substantial, but only a few well‐established, standardized, validated methods can be applied across the range of relevant foods. Furthermore, the prolonged period between infection and clinical symptoms (from several days for Cryptosporidium to several years for Echinococcus spp.) means that traceback studies are very difficult, if not impossible. Nevertheless, our knowledge of the domestic animal lifecycle (involving dogs and sheep) for Echinococcus granulosus means that this parasite is controllable.
For Echinococcus multilocularis, for which the lifecycle involves wildlife (foxes and rodents), control would be expensive and complicated, but could be achieved in targeted areas with sufficient commitment and resources.4 The successful "control" of Trichinella in commercial pig production offers a compelling example of overcoming this substantial commitment of resources for achieving risk reduction and positive public health outcomes. Commercial pig production in the U.S., since the mid-20th century, has seen a dramatic decline of this dangerous, medically important parasite. Most pig-derived meat offered for sale in the U.S. is free from this parasitic worm. For the years 2011–2015, in the U.S., on average there were 16 reported cases of trichinellosis per year.5
Quantitative risk assessments have been described for Toxoplasma in meat. However, for T. gondii, Cryptosporidium, and Cyclospora as fecal contaminants, development of validated detection methods, including survival/infectivity assays and consensus molecular typing protocols, are required for the development of quantitative risk assessments and efficient control measures. These challenges in detection and transmission are an impediment to the traceback methods and techniques used by CDC and FDA when seeking to identify the root cause of a disease outbreak. The 2023 Cyclospora outbreaks in the U.S. offer a case study in failed traceback.
Cyclospora cayetanensis
Cyclospora cayetanensis is a type of protozoa, which is a tiny, spherical, single-celled organism. It is transmitted when people ingest contaminated feces, typically through exposure to contaminated food or water. It can be spread only through human excreta, unlike E. coli and Salmonella, which can also be spread from animal fecal matter. The clinical picture of cyclosporiasis can be indistinguishable from that of giardiasis, cryptosporidiosis, and isosporiasis. Cyclospora infection can cause severe abdominal pain, watery diarrhea, nausea, vomiting, body aches, and fatigue. Symptoms can develop between 2–14 days after exposure.6 Although symptoms can be severe enough to send people to the hospital, it is rare for people to die from Cyclospora infections.
Cyclosporiasis is caused by ingestion of oocysts of Cyclospora cayetanensis, a parasite that was first recognized in the late 1970s. Foodborne outbreaks of cyclosporiasis have been reported in the U.S. since the mid-1990s. There are no reports of this disease in the U.S. prior to 1995.6 According to CDC's National Outbreak Reporting System (NORS), for the interval from 2000–2017, there were 39 reported foodborne outbreaks of cyclosporiasis that occurred in the U.S.; the total case count for those outbreaks over the 17 year interval was reported as 1,730 individuals. For that 17-year interval, the outbreaks occurred during eight different months. The peak months were May, June, and July. Cyclospora infections generally increase in the summer months, but can occur at any time. The NORS data also show outbreaks occurring in most of the contiguous states. It is noteworthy that for this 17-year interval, no outbreaks were reported for either Alaska or Hawaii. The NORS findings confirm that infections have been traced to fresh produce such as berries, sugar snap peas, basil, green onions, cilantro, and lettuce.6,7
In 2023, in the U.S., there was a dramatic increase in the number of confirmed cases of cyclosporiasis. According to CDC, as of October 24, 2023, 41 jurisdictions, including 40 states and New York City, have reported a total of 2,272 laboratory-confirmed cases of cyclosporiasis. The agency's October report noted that there had been an increase of 315 cases since its last update on August 31, 2023. The agency also confirmed that there were a total of 186 hospitalizations resulting from these Cyclospora infections. It is important to note that the infected individuals had not traveled outside of the U.S. during the 14 days before they became ill, indicating that exposure to the source of the infection occurred domestically.
The true number of people sick with cyclosporiasis during the 2023 outbreak is likely much higher than the number reported, as some people recover from the illness without medical care and are not tested for Cyclospora. Outbreak data for this episode show that Texas and New York reported between 201 and 360 cases. They were followed by Florida, Colorado, Virginia, and Illinois, where the reports of illness were in the range of 101–200 cases. Ten states had no reports of cyclosporiasis in 2023. These findings suggest that Cyclospora cayetanensis is now endemic in the U.S.
As of the time of publication, the root cause (food source) of the 2023 outbreak has yet to be identified.8 According to FDA and CDC, identifying the particular food item or other ingredient that has caused an outbreak of cyclosporiasis can be very challenging. CDC and other agencies are working to develop and validate molecular typing methods that can distinguish among different strains of Cyclospora. It is anticipated that these new methods will enable more efficient and effective traceback in the event of future outbreaks.
Prevention and control of the spread of Cyclospora, according to CDC guidelines, encompasses thorough handwashing, washing utensils used in food preparation, and thoroughly washing all fresh fruits and vegetables before cooking and/or refrigerated storage. Other food safety experts say there is no evidence that washing produce will effectively remove the parasite.
On the farm, good agricultural practices (GAPs) are critical for preventing food crops coming in contact with human waste. Light-based technologies, such as high-intensity pulsed light, may also be an effective control intervention. Atmospheric plasma might also have a future in this space. The technology is ideally suited for use in surface decontamination of fruit, vegetable, and meat products. In the processing of fresh fruits and vegetables, it is difficult to define and then validate chemical-aided washing as a critical control point targeting foodborne parasites, due to the resistant nature of these complex organisms. Bates and others have reported using high-power ultrasound for eliminating Cryptosporidians in potable water supplies.9 There is a possibility of combining chemical-aided washing with high-power ultrasound to combat Cyclospora on fresh produce. A similar technique has been studied for use in washing leafy greens. It is also conceivable that low-energy electron beam (LEEB) technology may prove an effective control strategy for certain types of food. Ultimately, and because Cyclospora are associated exclusively with human waste, a key in preventing and controlling its spread is good hand hygiene. Effective handwashing will break the fecal-to-oral route of transmission.
Toxoplasma gondii
Toxoplasma gondii is an interesting organism in the context of foodborne disease and food safety. Public health agencies report that as much as one-third of the world's population are infected with this parasite, and it often goes unrecognized, as most patients are asymptomatic. T. gondii may be acquired by eating contaminated raw or poorly cooked meat, especially pig and sheep.5,11 There are also reports of T. gondii infections occurring as a result of eating poorly washed vegetables or vegetables that have been washed with T. gondii-contaminated water.
The sexual cycle of T. gondii occurs in the Felidae, particularly widespread in domestic cats (Figure 1).4,10 These carnivorous final hosts become infected by eating flesh contaminated with oocysts or other infective stages in the parasite's lifecycle. Rats and mice are reported as important vectors in the transmission of the disease in cats. Herbivorous animals acquire the infection by ingesting oocysts in food or water contaminated by infective cat feces. Oocysts of T. gondii are reportedly able to survive in the environment for up to a year.10 Census data from the American Veterinary Medical Association for 2023 report that there are more than 58 million pet cats in the U.S., and anywhere between 30 million and 100 million stray or feral cats.11 The tons of fecal material generated annually by this enormous feline population is incalculable. Moreover, the impact of this hazardous material in the environment (by way of landfill) does not appear to be very well-studied or documented. Its possible threat to agriculture and food production appears to remain an open and unanswered question.
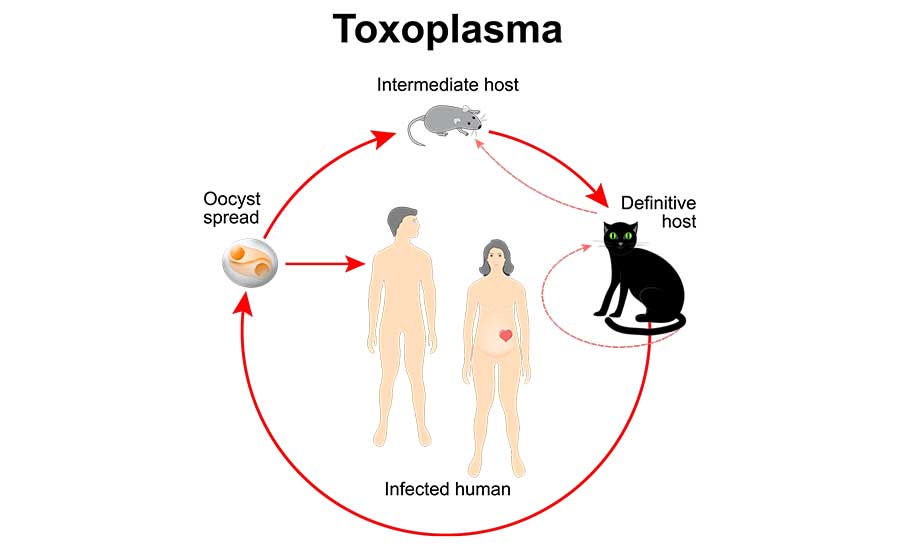
Toxoplasmosis, the disease caused by T. gondii, is most common in warm, moist areas, and is worldwide in distribution. It has been reported from humans, pigs, sheep, cattle, dogs, cats, and other domestic animals. It is likely the most common infectious pathogenic animal parasite of humans, occurring in 30 to 50 percent of the human population. Serologic evidence indicates that 90 percent of the population of some areas are affected. Symptomology of the infection varies widely, but most cases of toxoplasmosis are asymptomatic.10 When symptoms are reported, they are similar to influenza. The main population at risk of clinical disease are pregnant women and those with compromised immune systems. Infection during pregnancy has been reported to result in miscarriage or a stillborn infant.10 CDC reports that more than 40 million people in the U.S. may be infected with the Toxoplasma parasite.10
Preventing food sources from contact with feline feces, or water that has been contaminated by it, is the best way of breaking the cycle of transmission. Cooking meat for 45 seconds at an internal temperature of 55 °C (131 °F) or for 6 seconds at an internal temperature of 63 °C (145 °F) or higher will kill the parasite. Freezing meat for 3 days at –20 °C is also effective at killing this organism.11 Caution should be exercised when washing fresh fruits and vegetables, especially those to be eaten raw, and preventive measures taken to ensure that the wash water is free from contamination by cat feces.10
It is important, from a risk perspective, to consider that the source of this deadly disease-causing parasite is inadvertent consumption of feline feces. The lifecycle of T. gondii is dependent on its definitive host, which is domestic or wild cats. Humans are susceptible to infection by its oocysts, which are shed in the cat's feces.5,10 Likewise herbivores can become infected by eating grass or other feedstuffs contaminated by cat feces. The parasite then becomes established in the muscle tissues of those animals and remains for the balance of their life. Although foodborne infections in humans are rare, it could occur through eating raw vegetable from a home garden that has been contaminated with cat excrement. Raw or undercooked meat derived from both pork and lamb are also reported as foodborne sources of the parasite. Toxoplasmosis is typically asymptomatic or associated with mild flu-like illness, but the infection can be serious or life-threating in immunocompromised individuals. The unborn fetus is also at risk due to the parasite's ability to cross the placenta. Control of T. gondii involves thorough cooking of foods that are at risk of contamination. Washing fresh fruit and vegetables has not been shown effective in removing the parasite. It is imperative to keep cats away from all food sources and observe the strictest hygienic practices when handling and cleaning cat litter boxes. Hand hygiene is an imperative in preventing the spread of this parasite to humans.
Taenia solium and Trichinella spiralis (nematodes)
Porcine-transmitted, medically important parasites are well known and documented. These include Taenia solium, Trichinella spp., and Toxoplasma gondii.5,10 Of all the pork-borne parasites, none are better known than Trichinella spp. Humans can become infected with this parasite by consuming raw or undercooked meat containing the larvae of Trichinella. In the U.S., humans most often acquire the disease by eating raw or undercooked pork, wild boar, or bear meat. In other parts of the world, there are reports in the medical or public health literature of horse flesh as a source of transmission for this nematode to humans. Roast, smoked, or cured ham, sausages, and bacon are just a few examples of the kinds of pork products frequently implicated in the transmission of this disease.
Public health officials recommend heating pork or other of the Trichinella-harboring meat products to an internal temperature of 71 °C (160 °F), followed by maintaining that internal temperature (worst-case heating location) for a minimum of 2 minutes. Between 1947 and 1967, CDC surveillance data show a precipitous overall decline in pig-mediated trichinosis in the U.S.12 The observed decline of this pernicious, parasitic disease in humans was due, in large measure, to changes in on-farm practices related to housing, feeding, and implementation of improved standards of care for swine prior to slaughter. Public health officials and veterinarians advised farmers against feeding the animals garbage, and also of the need for preventing the pigs from exposure to rats—an important vector in the transmission of the disease. Concurrent with these on-farm developments, public health officials, home economics teachers, gym teachers, and librarians across the land were enrolled in an unprecedented campaign to educate the public of the risks of eating undercooked pork.
For the period 1967 to roughly 1979, CDC surveillance data show large spikes in reported cases of trichinellosis in the U.S.12 The case load levels observed during this interval were consistent with those observed in the 1950s. This period of increased disease activity corresponded with the increased uptake and use of microwave ovens by American consumers. According to J. Carlton Gallawa, author of The Complete Microwave Oven Service Handbook, prior to 1967, only about 14 percent of U.S. households were using microwave technology. However, during the interval 1975–1978, more than 60 percent of U.S. households owned microwave ovens. During this time frame, the number of cases of trichinellosis went from less than 100 reported cases in 1967 to nearly 300 cases in 1975. This trend continued until about 1980, when the U.S. Department of Agriculture (USDA) issued guidelines for cooking pork in a microwave oven (internal temperature of greater than 170 °F). With the advent of these cooking guidelines, the trend line for the number of trichinellosis cases fell off sharply, so that they were again consistent with the observed caseloads prior to 1967. These data are at least suggestive of a causal relationship between the microwave oven and the incidence of trichinosis.
Depending on the classification used, there are several species of Trichinella: T. spiralis, T. pseudospiralis, T. nativa, T. murelli, T. nelsoni, T. britovi, T. papuae, and T. zimbabwensis, all but the last of which have been implicated in human disease. Adult worms and encysted larvae develop within a single vertebrate host. A second host is required to perpetuate the lifecycle of Trichinella. In domestic animals, the cycle most often involves pigs and rodents, but other domestic animals such as horses can be involved.5
Trichinella infections may be asymptomatic. Intestinal invasion by the parasite can be accompanied by gastrointestinal symptoms (diarrhea, abdominal pain, vomiting). Larval migration into muscle tissues can cause facial edema, conjunctivitis, fever, myalgias, rashes, and peripheral eosinophilia. Occasional life-threatening manifestations include myocarditis, central nervous system involvement, and pneumonitis. Larval encystment in the muscles causes myalgia and weakness, followed by subsidence of symptoms.13
Trichinellosis is an increasingly common medical problem in the U.S., especially in the Southwest and other areas of heavy emigration from endemic areas or in populations with significant travel to these areas.14
Anisakis spp.
Parasitic foodborne infections are by no means limited to terrestrial animals. Anisakis spp. are important foodborne disease-causing agents found in marine fishes. Anisakiasis, or herring worm disease, is a parasitic disease caused by nematodes (roundworms). The larvae of the Anisakidae are commonly found in the mesenteries and peritoneum of marine fishes. Anisakiasis is acquired by eating contaminated raw, pickled, or lightly salted fish such as herring, haddock, cod, mackerel, bonito, salmon, and pike (Figure 2).14 Undercooked squid have also been implicated as an etiologic agent of this disease.

Symptoms are reported as similar to those of patients presenting with peptic ulcers or tumors and include nausea, vomiting, bloody diarrhea, and fever.14 Live herring worms can be up to 2 centimeters in length. Once ingested, they invade the intestinal wall and cause symptoms that mimic those of food poisoning, as described above. In most cases, the worm dies after a few days and the symptoms resolve.14 According to CDC, anisakiasis is rarely diagnosed because most people assume they merely suffered a bad case of food poisoning and do not seek medical diagnosis. The global incidence of the disease is relatively low and limited in large measure to Japan and Korea, where eating raw fish and squid are a culinary habit. However, with the trend in many western countries of eating sushi and related raw fish dishes, it is reasonable to expect an increase in the incidence rate of anisakiasis worldwide.
A 2020 study led by scientists at the University of Washington and published in the journal Global Change Biology found "…dramatic increases in the abundance of the parasitic nematodes Anisakis spp. and Pseudoterranova spp. in marine fishes and invertebrates."15 These researchers reported an astonishing 283-fold increase in the abundance of these nematodes since the 1970s.15 Their work infer implications for "the health of humans and marine mammals, which both can inadvertently eat the worms." The researchers do not suggest a definitive cause for the massive increase of Anisakis worms over the past several decades, but they opine that climate change, more nutrients from fertilizers and runoff, and an increase in marine mammal populations over the same period could be potential reasons.15
The pathology of anisakiasis involves the parasitic worm attaching to the wall of the esophagus, stomach, or intestine. However, the Anisakis worm cannot reproduce or live for more than a few days in a human's intestine. Humans are not its definitive host; however, the parasite can persist and reproduce in a number of marine mammals that serve as its intermediate host. The worms reproduce in the intestines of these animals and are released into the marine environment by way of the mammals' feces.14 The worms then infect other creatures, including certain fish and squid that are used as human food. Another important implication of this study, for food safety scientists, is that the massive, rising public health risk for those who consume raw or undercooked fish and squid is now known. Is this new information reconciled in your food safety plan or risk assessment?
Conclusion
Epidemiologic studies have shown that parasite contamination of food and food ingredients may occur at several points along the supply chain, starting from the use of contaminated water for irrigation and contact with animal and human excrement during cultivation, through contaminated harvesting equipment, and including unhygienic practices (such as open defecation) by farm workers in the production field or others handling the food material prior to consumption. Parasite transmission stages, like the oocyst of Cyclospora, tend to be robust and, therefore, likely to survive from contamination in the field, through harvesting, packaging, and until consumption. Foodborne parasitic diseases are often overlooked or neglected in food safety control schemes, even though they are known to pose a severe threat to human health. Parasitic disease in humans are notoriously difficult to detect, diagnosis, and treat. This simple truth may account for this class of foodborne disease-causing agents often being left out of the risk assessment equation.
Different types of parasitic diseases can be transmitted to humans by an assortment of food materials. Pork, freshwater crustaceans, sheep, fish, fresh fruit, and vegetables have all been implicated in disease outbreaks. Water and the environment have also been shown as viable, high-risk routes of human and farm animal infection. Food safety interventions, however, have been proven effective in controlling or limiting the public health and disease burden that are associated with certain foodborne parasitic diseases. The great success with the control of trichinellosis in the U.S. is an excellent example.
Many years ago, I posited this definition for food safety: "Food safety is the chemical, physical, or biological status of a food that allows its consumption without incurring an excessive risk of injury, morbidity, or mortality." The fulcrum about which this definition operates is the notion of risk. As much as we would like for the limits of the operation to approach zero, they seldom do. Simply put, risks are inherent to the production, distribution, and consumption of human food. There are regulatory risks, reputational risks (for the enterprise), and most importantly, there are risks to public health. It is the latter that we strive most ardently to control or eliminate. Of the three risk categories—chemical, physical, and biological—perhaps the most daunting in terms of control are foodborne pathogens, including parasites. Thus, it is incumbent that food safety scientist consider parasites in the context of their risk assessment and food safety plans.
Food safety is dynamic. We learn new things about new pathogens or new threats to the food supply almost on a daily basis. Who knew that Toxoplasma gondii was a killer, or that at least one-third of the people on the planet are infected with this parasite? Then there are the organisms of unknown status—do they or do they not cause human illness, and are they capable of transmission via food intended for human consumption? For example, we have clinical confirmation for ill patients that they have been exposed to Cyclospora, but then FDA and CDC cannot pinpoint the food or beverage source(s) that have caused the infection.
It also appears that climate change may be altering the geographic distribution of certain pathogenic microorganisms, both bacterial and parasitic, resulting in infections and food safety failures in areas where prior assessments of risk would not have predicted. Consider, for example, that prior to 1996 there were no reported cases of cyclosporiasis in the U.S., but as of 2023, the epidemiological data suggests that this parasite has become endemic in much of the country. The list of organisms of concern is growing, and so we must remain vigilant in our efforts to identify and control these new threats to the food supply and public health.
A 2023 report from Australia of foodborne infection involving the parasitic worm Ophidascaris robertsi is a chilling account.16 The worm was supposedly acquired as a result of exposure to "Warrigal greens" that were contaminated with the oocysts of Ophidascaris, a roundworm usually found in pythons. The pathology of this infection made medical news, as it was the first reported account of a parasitic worm crossing the blood-brain barrier and becoming established in a mammalian brain. In terms of risk assessment, Ophidascaris would likely rank inconsequential. The point here, however, is that food safety is dynamic, and we receive reports of new threats on a near-daily basis. Failing to consider medically important foodborne parasites in your risk assessment may prove to be a risky proposition.
References
- Yanet, F.-S., N.-F.F. Angel, N. Guillermo, and S.-P. Sergio. "Comparison of parasitological techniques for the diagnosis of intestinal parasitic infections in patients with presumptive malabsorption." Journal of Parasitic Diseases 41, no. 3 (September 2017) 718–722. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5555921/#:~:text=Among%20parasitological%20techniques%2C%20the%20direct,limitations%20to%20identify%20helminthic%20eggs.
- Scallan, E., P.M. Griffin, F.J. Angulo, R. Tauxe, and R.M. Hoekstra. "Foodborne Illness Acquired in the United States—Unspecified Agents." Emerging Infectious Diseases 17, no. 1 (January 2011). https://wwwnc.cdc.gov/eid/article/17/1/p2-1101_article.
- Gouvras, A. "Taenia solium, taeniasis and cysticercosis." BugBitten. BioMed Central. https://blogs.biomedcentral.com/bugbitten/2023/12/29/taenia-solium-taeniasis-and-cysticercosis/.
- EFSA BIOHAZ Panel. "Public health risks associated with food-borne parasites." EFSA Journal (December 4, 2018). https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2018.5495.
- U.S. Centers for Disease Control and Prevention (CDC). "Trichinellosis FAQs." September 4, 2020. https://www.cdc.gov/parasites/trichinellosis/gen_info/faqs.html.
- CDC. "Parasites—Cyclosporiasis (Cyclospora Infection)." August 24, 2023. https://www.cdc.gov/parasites/cyclosporiasis/index.html.
- CDC. "National Outbreak Reporting System (NORS)." https://wwwn.cdc.gov/norsdashboard/.
- Marler, B. "Nationwide Cyclospora outbreak hits 2,272 with no source identified." November 4, 2023. Marler Blog. https://www.marlerblog.com/case-news/nationwide-cyclospora-outbreak-hits-2272-with-no-source-identified/.
- Bates, D. and L. Keener. Personal correspondence and joint presentations 2015 on food industry application of high-power ultrasound.
- CDC. "Toxoplasmosis: General FAQs." December 1, 2020. https://www.cdc.gov/parasites/toxoplasmosis/gen_info/faqs.html.
- Osborn, J.F. "How Many Cats Are in the USA?" October 14, 2023. https://worldanimalfoundation.org/cats/how-many-cats-are-in-the-usa/.
- CDC. "Trichinosis surveillance, 1984." Morbidity and Mortality Weekly Report 35: 11SS–15SS. 1986.
- Kraft, R. "Cysticercosis: An Emerging Parasitic Disease." American Family Physician 76, no. 1 (2007): 91–96. https://www.aafp.org/pubs/afp/issues/2007/0701/p91.html.
- Food and Agriculture Organization of the United Nations (FAO). "Parasites in Foods: An Invisible Threat—Food Safety Technical Toolkit for Asia and the Pacific." 2020. https://www.fao.org/documents/card/en?details=CB1910EN/.
- Fiorenza, E.A., C.A. Wendt, K.A. Dobkowski, T.L. King, M. Pappaionou, P. Rabinowitz, J.F. Samhouri, and C.L. Wood. "It's a wormy world: Meta-analysis reveals several decades of change in the global abundance of the parasitic nematodes Anisakis spp. and Pseudoterranova spp. in marine fishes and invertebrates." Global Change Biology (March 19, 2020). https://onlinelibrary.wiley.com/doi/10.1111/gcb.15048.
- Magramo, K. "'Still alive and wriggling:' Doctors remove 3-inch parasitic worm from woman's brain in world first." CNN. August 29, 2023. https://www.cnn.com/2023/08/29/australia/australia-parasitic-worm-brain-scn-intl-hnk/index.html.
Larry Keener, CFS, PA, is President and CEO of International Product Safety Consultants. He is also a member of the Editorial Advisory Board of Food Safety Magazine.