
Image credit: AleksandarGeorgiev/E+ via Getty Images
Balancing Chemical and Biological Hazards With the Help of APAs to Safeguard Food Quality
Antimicrobial processing aids are preventive control interventions that are capable of reducing bacteria from the surfaces of foods at multiple steps throughout the supply chain
Beyond the delectable ingredients gracing our plates and filling our glasses, a concealed realm of antimicrobial food safety preventive control technologies diligently work behind the scenes to assure the safety and excellence of our culinary experiences. In this review, chemical sanitizers, disinfectants, cleaners, antimicrobial processing aids, and food additives used for pathogen control and shelf-life extension are discussed in a way that can be communicated to the workforce responsible for implementation of the processes. Insights are shared about the current thinking regarding safe levels of the respective chemicals in our foods, how they are regulated, and best practices for implementation.
Simple Guiding Principles
Chemicals incorporated into foods, applied to the surfaces of foods, or used to clean, disinfect, or sanitize food contact surfaces have one thing in common. They all have thresholds that, when exceeded, can cause harm. The methods to assess the risks and establish threshold tolerances for toxicity are far more advanced now than they were when Dr. Wiley conducted his "hygienic table trials," better known as "the Poison Squad." Nonetheless, the guiding principle of protecting the public from chemically adulterated foods is the same now as it was in the early 1900s when the original Pure Food and Drug Act, Meat Inspection Act, and Federal Insecticide Act were passed.
The use of antimicrobial chemicals in food production is currently regulated by three agencies in the U.S.:
- The Food and Drug Administration (FDA)
- The U.S. Department of Agriculture Food Safety and Inspection Service (USDA-FSIS)
- The Environmental Protection Agency (EPA).
While FDA takes the lead for food chemical safety,1 the three agencies work together via a memorandum of understanding (MOU) adopted to accomplish these responsibilities (Table 1).
FDA is responsible for ensuring that foods do not contain illegal chemical contaminants. USDA-FSIS is specifically responsible for ensuring the wholesomeness and safety of meat, poultry, egg, fish, and fish products. EPA is responsible for administering and enforcing the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) and the Toxic Substances Control Act.
Sanitizers and Incidental Contact in Food Processing
The words sanitize, sanitizer, and incidental contact are widely used in the public domain, trade magazines, and peer-reviewed journal articles. Sanitizers and disinfectants are two types of antimicrobial pesticides. With the exception of cleaned whole shell eggs, neither are intended for direct application to foods or beverages.
The public domain can be confusing when it comes to the definition of sanitizers, incidental contact sanitizers, and disinfectants. The word "sanitation" comes from Latin "sanitas" (health). EPA defines incidental food contact surface sanitizers as pesticides used to kill bacteria and other microorganisms that may be present on surfaces used to prepare, serve, transport, and store food.
In the U.S., chemical germicides formulated as sanitizers are regulated in interstate commerce by EPA's Office of Pesticides Program (Antimicrobials Division) under FIFRA. EPA requires manufacturers of sanitizers, disinfectants, or chemical sterilants to test formulations by using accepted methods for microbiocidal activity, stability, and toxicity to animals and humans. The manufacturers submit these data to EPA along with proposed labeling. If EPA concludes that the product can be used without causing "unreasonable adverse effects," then the product and its labeling are registered, and the manufacturer can sell and distribute the product in the U.S.
Food processors and retail establishments that use registered incidental contact sanitizer (ICS) products must explicitly follow the labeling directions on each product. Failure to follow the specified use-dilution, contact time, method of application, or any other condition of use is considered a misuse of the product and subject to enforcement by FDA, USDA-FSIS, and EPA, which can include product recall, fines, and civil penalties. Therefore, effective control programs including work instructions for mixing/diluting the sanitizer, keeping monitoring/verification records, and implementing corrective action plans must be in place to prevent misuse of ICS products.
As previously stated, the registered label instructions must be explicitly followed. The following are examples of general factors that must be controlled:
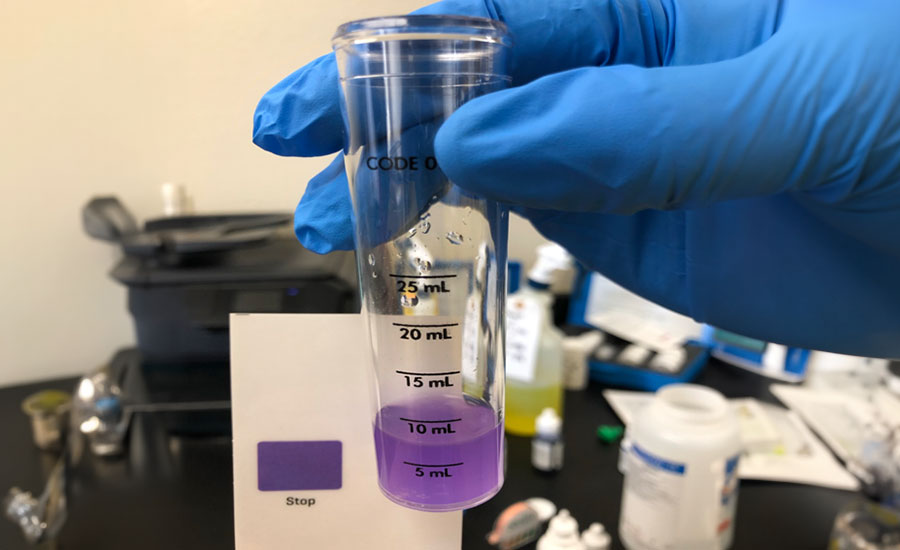
- Concentration: The presence of too little sanitizer will result in an inadequate reduction of microorganisms, while too much can be toxic. It is necessary to have specific testing kits to measure and record chemical sanitizer concentrations.
- Contact time: In order for the sanitizer to kill microorganisms, the cleaned surface must be in contact with the sanitizer for the recommended length of time.
- Air drying: After applying the ICS, the equipment and/or utensils must be allowed to air dry before use.
Trained and qualified individuals are required to prepare, monitor, and verify ICS procedures before each use and after each application to assure regulatory compliance and optimal efficacy. Mixing concentrated sanitizer chemicals with potable water to achieve the approved no-rinse ppm dilution target specified on the label requires precise measurement and testing before use. To avoid the risk of improper sanitizer preparation, food establishments often use ready-to-use sanitizers, which are pre-diluted to the registered/approved incidental contact ppm levels and are available in solution and pre-moistened disposable wipes. Records should be reviewed daily and corrective actions implemented, as needed.
When applied within labeling/EPA registration thresholds and use parameters, the amount of residual sanitizer chemicals that ultimately contact food is so low after adequate draining or air drying, that FDA has determined the ICS residue does not pose a consumer health risk.
EPA uses dietary exposure models2 to assist with establishing exposure limits to components of sanitizing solutions. Sanitizer use is codified under the FFDCA Section 408 by way of three different use scenarios, associated with 40 CFR 180.940(a), 40 CFR 180.940(b), and 40 CFR 180.940(c) that includes the ready-to-use, end-use concentration/threshold or exposure limits (ppm).
Sanitizers and disinfectants, like cleaners, are toxic above defined thresholds. Unlike cleaners, sanitizers and disinfectants make antimicrobial claims and absolutely require pre-market review and registration as antimicrobial pesticides with specific use regulations under the authority of EPA.
Foods are considered adulterated if food comes into contact with a food contact surface that was sanitized with a concentration exceeding the registered incidental contact threshold, or if food comes into contact with "pooled" sanitizer on the equipment that has not properly drained or air dried, even if the ppm of the sanitizer is within the labeled tolerance for incidental contact (Figure 1).
FDA defines the term "sanitize" as meaning to adequately treat cleaned surfaces by a process that is effective in destroying vegetative cells of pathogens, and in substantially reducing numbers of other undesirable microorganisms, but without adversely affecting the product or its safety for the consumer.
Training to prevent chemical contamination should go beyond just those involved with mixing, titrating, and applying sanitizers. All associates on a processing line or in a foodservice kitchen in which sanitizer chemicals are used should understand the proper use of sanitizers. For example, during production, a food handler once poured a container of glove dip sanitizer onto a belt with finished product on it. The product could not be reconditioned, and the line was shut down for re-cleaning. The food handler revealed that they thought they were assisting with the process because they had observed the solution being applied to the work/food contact surfaces at scheduled breaks during operation. The threshold was not out of compliance, but the condition of use was not incidental contact.
When the registered label directions for use are followed and the concentration is less than the threshold, the residual amount of sanitizer chemical residue still on the food contact equipment after draining/air drying that contacts food must be incidental and cause no harm.
Cleaning Products in Food Processing
Unlike EPA-registered incidental contact sanitizers, cleaning compounds have zero tolerance for incidental contact with foods. In addition, there is no requirement for pre-market federal government agency review and approval for food processing cleaning chemicals in the U.S., unless an antimicrobial claim is made. Therefore, the responsibility of assessing and managing the risks associated with cleaning products for specific uses in food manufacturing falls on the manufacturer and user.
Prior to February 1998, USDA-FSIS provided food processors and customers an agency program for reviewing and approving non-food compounds3 and proprietary substances, including FDA 21 CFR, for appropriate use and ingredient and labeling review. Although the program was developed for the meat and poultry industry, all food sectors had access to the program and could utilize it to source appropriate products that were suitable and scientifically validated for safe use. Cleaning products, for example, were divided into nine category codes based on use (Table 2).
USDA-FSIS coordinated in-depth reviews by USDA, EPA, FDA, and the Occupational Safety and Health Administration (OSHA) before approving a non-food compound, and they maintained a list of approved products and their specific use criteria in the National Sanitation Foundation (NSF) White Book. The FSIS program provided assurance to food processors, customers, and food regulators that use of the compounds and substances on the list would not result in the adulteration or contamination of food, provided they were used as labeled. The USDA program was terminated with the implementation of Hazard Analysis and Critical Control Points (HACCP), reflecting a change in agency philosophy from prescriptive to performance-based safety systems.
The non-food compound review criteria used previously by FSIS4 is available to the public. For example, a food contact surface cleaning product that makes no antimicrobial claims should not have an expected human single oral LD50 of < 10 mg/kg.
In addition to technical supporting documentation for the safe use of cleaners provided by the cleaning company, independent analysis may include a review of safety data sheets (SDS). Acute toxicity estimates (ATE) for the classification of a cleaner substance can be derived by using the percentages of each known chemical in the solution and the corresponding LD50/LC50 values, where available. However, chemicals present at levels less than 1 percent of the mixture and some proprietary chemicals may not be included in the SDS.
The NSF White Book provides a continuation of the USDA Non-Food Compound Product Approval and Listing program. For a product to be reviewed, the complete formula (including all proprietary substances) must be provided to NSF. Many food processing cleaning product companies and their respective products are registered with NSF, and many are not.
The FDA Food Code defines cleaners as "poisonous or toxic materials" that are not intended for ingestion. They are for cleaning inanimate hard surfaces; they are not for washing or treating vegetables or fruit. Even when "green" cleaners5 are used on hard food contact surfaces, they must be thoroughly rinsed off, so that no residual chemical remains that might be transferred from the cleaned equipment or utensils onto food.
In addition, if the cleaning chemicals are not thoroughly rinsed off of food contact equipment, then the "carryover" chemical(s) can negatively interact with subsequent incidental contact sanitizer chemicals, resulting in diminished efficacy and/or inactivation of the active ingredient. The right slide of Figure 2 shows carryover cleaning chemical residue that can be seen as yellowish fluorescence on the underside of the stainless steel support for the belt's wear strip.

Antimicrobial Food Processing Aids
Antimicrobial processing aids (APAs) are preventive control "treatments" and/or "interventions" that are capable of reducing bacteria from the surfaces of foods at multiple steps throughout the processed foods supply chain. They benefit public health via reducing pathogens, and they also benefit quality and sustainability by reducing spoilage organisms and decreasing food waste. APAs have been safely used for many years in the meat and poultry industry and have been gaining value in other food manufacturing commodities and retail foodservice operations.
APAs are not sanitizers or disinfectants. They are a subgroup of food additives intended to be applied directly onto foods under set conditions without the need for a potable water rinse. Although some of these aqueous solutions may contain chemicals that are also used in sanitizers, APAs are not antimicrobial pesticides regulated under the authority of EPA or FIFRA labeling. However, like incidental contact sanitizers, each APA has a defined maximum concentration threshold and specific use condition.
FDA reviews APAs and other substances intended for use in the manufacturing or packaging of foods via the FDA Food Contact Substance Notification (FCN) Program.6 The codified thresholds for regulation safety standards are generally the same for APAs as they are for all food contact articles (21 CFR 170.39),7 whether subject to the petition or FCN process. For example, substances submitted through the FCN process must not be a carcinogen in humans or animals or a potent toxin. Based on the substance's chemical structure, there must be no reason to suspect that the substance or any impurities contain a carcinogen or potent toxin. If the Food Contact Substance (FCS) contributes to a cumulative dietary concentration greater than 200 parts per billion [0.6 milligrams (mg)/person/day] in the daily diet for a biocide or 1 part per million (3 mg/person/day) in the daily diet for a non-biocide substance, then the substance must be submitted through the petition process.
In the U.S., Canada, and other countries, APA solutions do not need to be declared on finished product labels, and they are often overlooked when discussing food antimicrobials. APAs meet the definition of a "processing aid"8 because they are a treatment or intervention that will drip off, evaporate, break down, or otherwise leave no significant residue on the food and have no lasting technical effect, and therefore are exempt from labeling8 when applied as approved for use.
APAs are made of various food additives and/or Generally Recognized as Safe (GRAS) ingredients that FCS efficacy validation studies have shown to have antimicrobial properties and the FDA has reviewed without objection and listed in the FCS Inventory.6 Each APA includes a unique FDA assigned Food Contact Notice Number, the Food Contact Substance (FCS), the manufacturer, the effective date, the intended use, the limitations/specifications, the environmental assessment, and the FDA/EPA Finding No Significant Impact Report.
FCNs are effective only for the listed manufacturer and its customers. Other manufacturers must submit their FCNs for the same food contact substance and intended use. A notification is not required for a food contact substance that is GRAS or prior sanctioned for its intended use in contact with food.
APAs are regulated in further processing facilities by FDA and FSIS. They are inventoried under FDA's Food Contact Substance Notification Program and FSIS Directive 7120.1: Safe and Suitable Ingredients Used in the Production of Meat, Poultry, and Egg Products.9
FDA provides up-to-date APA "Safe Harbor" reference documentation for processors and regulators. The Inventory of Effective Food Contact Substance Notifications database lists effective premarket notifications for food contact substances that have been demonstrated as safe for their intended use. The presence of a substance on this list indicates that FDA has approved only certain intended uses and use conditions. FDA updates the list by adding new FCNs and removing them when they are no longer safe. FDA provides the public with decision-making documentation for the safe use of effective food contact substances and their environmental impact10 that are good resources for food safety and sustainability plans.
APA technologies are often factored into supplier risk ranking models and added to risk-based supply chain preventive control programs/purchasing agreements to enhance the reliability of achieving food safety objectives. In some instances, APA systems have been implemented as temporary safeguards until major facility and equipment sanitary design improvements could be made. However, antimicrobial agents, including APAs that meet regulatory limits for food contact substances, do not have the log reduction power of thermal, electron-beam, or high-pressure pasteurization. An APA alone at the end of the process cannot be relied upon to eliminate pathogens. APA interventions should be combined into a multi-hurdle integrated food safety system with other best practices, such as supplier approval programs, Good Agricultural Practices (GAPs), sanitary dressing procedures, Sanitation Standard Operating Procedures (SSOPs), sanitary design of equipment, sanitary design of facility, Good Manufacturing Practices (GMPs), and Hazard Analysis and Critical Control Points (HACCP).
Pressure spray and drain delivery systems for APAs reduce bacteria loads from the surfaces of foods by physical displacement, as well as chemical inactivation. In addition, one-pass spray systems do not pose the risk of cross-contamination like dip/drench common pool systems do. However, hydrophobic food surfaces (Figure 3) pose spray system challenges to achieve full surface coverage and contact/cling time to inactivate the target organisms.
Food surface, solution chemistry, and delivery system design are key factors to maintain enough contact time for efficacy. To achieve adequate contact time, products with hydrophobic surfaces should be submerged.

Figure 3 shows how less than approximately 30 percent of a whole tomato surface is covered after being thoroughly sprayed with an organic acid APA solution containing a fluorescent food-grade tracer (B2) for better visualization of the solution surface tension and lack of coverage and cling time.
Food manufacturing, foodservice, and retail buyers should have insight into supplier preventive controls and verify that the antimicrobial agents used are effective, approved for domestic use, and internationally harmonized.
It should be noted that APAs do not inhibit pathogen outgrowth during the food's shelf life (no residual lasting effect). Therefore, very low levels of any remaining pathogens can multiply on products to infectious dose levels (Table 311) during transport and storage, under the right conditions. Therefore, antimicrobial ingredients with a lasting effect are often formulated into processed foods to inhibit the outgrowth of pathogens and spoilage organisms during storage.
For more information on changes to FDA's FCN, see the article in Food Safety Magazine, "FDA Amends Food Contact Notification Regulations."12
Direct and Indirect Antimicrobial Food Additives and GRAS Substances
Direct food additives that provide technical effects to the final food product must be listed on the food label by their common (or usual) name, as per 21 CFR Part 172. An example of a direct food additive could include microbial inhibitors that are formulated into products to limit outgrowth of certain bacteria and/or to extend shelf life.
Adding validated microbial inhibitors into products, when possible, is an important part of a multi-hurdle approach to microbiological hazard process control. In processed meats, especially ready-to-eat deli meats that have a long shelf life and high water activity, adding inhibitors in the formulas has been an industry best practice since the 1980s. Best-in-class meat processing companies and supply chain private-label distributors have required specific antimicrobial ingredients and percentages connected to certain groups of validation studies and models associated with certain outgrowth maximums over certain time periods and temperatures. The types of validated inhibitors have changed over the years (mostly to more natural ingredients), but their use in the meat industry is a standard, and labeling them continues to be widely accepted.
Acidified sodium chlorite (ASC) is an example of an indirect antimicrobial food additive that meets the definition of a processing aid under specific regulatory restrictions for its use in different products.
For example, the regulations treating raw agricultural commodities (RACs) with acidified sodium chlorite (ASC) solutions require a subsequent "intervening event," such as a potable water rinse, blanching, cooking, or canning, per 21 CFR 173.325(e). In 21 CFR 173.325(g), when ASC is applied onto specific processed leafy vegetables, the application must be preceded by a potable water rinse and followed by a 24-hour holding period before consumption. However, 21 CFR 173.325 also codifies its use on raw poultry, red meat, and seafood without rinsing/removing the chemical.
Regulation of antimicrobial additive intervention uses in U.S. food processing facilities fall within FDA's authority under § 409 of the Federal Food, Drug, and Cosmetic Act. Included within the meaning of the term "food processing facility" are those facilities operating under the Federal Meat Inspection Act, the Poultry Products Inspection Act, and the Egg Products Inspection Act. Also included are fish processing operations, commercial fishing vessels, and retail food establishments. However, when antimicrobial treatments are applied to RACs that are not processed, then such treatments are considered pesticide chemical applications whereby EPA has primary authority. Such applications are subject to a pesticide registration under FIFRA and/or an exemption from the requirement of a tolerance under § 408 of the Federal Food, Drug, and Cosmetic Act.
Antimicrobial preventive controls are important in post-harvest handling and processing operations for fresh and frozen fruits, vegetables, and herbs. A recent study by the European Food Safety Authority13 reported that the most relevant microbial hazards associated with such products are L. monocytogenes, Salmonella spp., human pathogenic E. coli, and enteric viruses. The study linked multiple outbreaks in the EU to water used during post-harvest handling. The panel confirmed that improper water management processes can accumulate all biological hazards, potentially resulting in batch-to-batch cross-contamination. The panel stated that an appropriate water management strategy, including validation, operational monitoring, and verification, must guide the implementation of water disinfection treatments. The panel said that contamination of water used in post-harvest handling and processing of fresh and frozen fruit, vegetables, and herbs is a global concern.
Contaminated wash water is also a concern in U.S. foodservice operations since fresh produce can be a vehicle for several pathogenic microorganisms leading to foodborne illnesses. Since there is no kill step, preventive controls must be in place. Researchers have studied the application of acidic and near-neutral hypochlorous acid14,15,16 (also referred to as electrolyzed water) by a process mimicking that used in a restaurant or foodservice operation. Acidic electrolyzed water with free chlorine of 40 ppm significantly reduced E. coli O157:H7 in wash or chilling solutions, compared to tap water.
Use of chlorine is authorized by the U.S. National Organic Program guide18 for use in water used in direct post-harvest crop or food contact at levels approved by FDA and EPA. However, in all circumstances following such permitted use, organic foods must be rinsed with potable water that does not exceed the maximum residual disinfectant limit for chlorine as defined under the Safe Drinking Water Act, as required by the Organic Food Production Act [7 U.S.C. 6510(a)(7)].
RAC handlers and food processing facilities should keep in mind that consumer concerns about synthetic additives have grown with increased awareness of health and food safety associated with harsh chemicals. Some European countries have banned chlorine due to its formation of hazardous byproducts in wastewater, including cytotoxic and genotoxic substances. The bans have prompted the exploration of alternative, greener technologies for vegetable preservation, aligning with the rising demand for natural antimicrobial additives.
Ozone is a secondary direct additive (21 CFR 173.368) and has been recognized as a "greener or more natural" antimicrobial agent for vegetable storage, washing, and processing. The USDA National Organic Program (NOP) has approved ozone as a "synthetic substance allowed for use in organic crop production" with specified conditions of use (7 CFR 205.601). The NOP has also approved ozone use "in or on processed products labeled as "organic" or "made with organic" in 7 CFR 205.605.
In 2019, the French Agency for Food, Environmental, and Occupational Health and Safety (ANSES) proposed extending ozone in water as a technological aid for washing ready-to-use salads. Researchers found promising environmental and product quality results. The authors reported that the efficacy against a broad spectrum of pathogens was dependent on several intrinsic and extrinsic factors such as microbial species and strain, ozone concentration, contact time and temperature, and delivery method (i.e., agitation, soaking, spraying, or bubbling). Some researchers and potential users believe that too many factors influence ozone technology and that it is not a robust industrial microbial intervention.
Peracetic acid (PAA) is another secondary direct food additive (21 CFR 173.370). PAA has advantages over ozone because it is less reactive to organic matter (especially protein) and can be utilized over wider temperature and pH ranges. It can also be used with hard water.19 PAA results from the combination of acetic acid and hydrogen peroxide. PAA has a complex regulatory status because several federal agencies have their own specific areas of statutory jurisdiction. Sections 40 CFR 180.1196 and 40 CFR 180.1197 establish the conditions for an exemption from the requirement for a tolerance for peroxyacetic acid (PAA) and hydrogen peroxide, respectively.
There are currently 120 PAA FCNs listed in the FDA Inventory of Effective Food Contact Substance Notifications. Specifically, 21 CFR 173.315(a) outlines wash water use limitations when PAA includes a stabilizer, and 21 CFR 173.370 codified the additive as approved for use as an antimicrobial agent on meat carcasses, parts, trim, and organs in accordance with current industry practice (Table 4). In addition, the current USDA NOP regulations permit the use of PAA as an antimicrobial for use in wash water and rinse water for raw and processed fruits and vegetables and meat and eggs, according to FDA limitations.20
The reason for PAA's efficacy in reducing bacterial loads is its specific capability to penetrate the cell membrane. Once inside the cell, PAA plays a role in denaturing proteins, disrupting cell wall permeability, and irreversibly disrupting enzyme systems by oxidizing bonds, which destroys the microorganism. The end products of PAA oxidation are acetic acid and water.
In addition to direct application of approved chemicals to reduce micro loads, certain processing aids exercise their antimicrobial prowess more discreetly. Diatomaceous earth, for example, is a natural filtering agent prevalent in beverage processing, and also in meat processing where recirculating brine is used. It functions like a microscopic bed of nails, screening, piercing, and neutralizing bacteria in transit.
For a better understanding and clarification of sanitizers and disinfectants used in foodservice, see the Conference for Food Protection's Guide for Washing and Crisping Whole Raw Fruits and Vegetables at Food Establishments.21
Premarket Review and Safe Harbors
For the most part, APAs and any other substances intentionally added to foods and beverages are subject to premarket review and approval by FDA, as previously stated. One exception is the replacement of the premarket affirmation process for GRAS ingredients with a GRAS Notification procedure. Congress crafted this exemption to be used infrequently, primarily to keep common ingredients like spices on the market without an onerous approval process. GRAS Notifications22 range from dried whole goat milk for non-exempt infant formula to the antioxidant pyrroloquinoline disodium salt for drinks and beverages. Of the approximately 1,200 GRAS Notifications in the database in 2023, 90 percent had corresponding FDA no-objection letters, 8 percent were pending determination, and 2 percent did not provide the basis for GRAS determination.
FDA reassesses GRAS and food additive ingredients on an ongoing basis and exercises authority to revoke an ingredient's GRAS or food additive status and prohibit its use in foods when ingredient data raises safety concerns. A recent example of a revoked food additive is brominated vegetable oil (BVO). BVO had GRAS status until 1970, when FDA recategorized it as a food additive. In November 2023, FDA revoked authorization of its use in food entirely after gathering enough data to show its bioaccumulation and toxic effects on the thyroid.
USDA-FSIS also provides "Safe Harbor" APA use guidance via FSIS Directive 7120.1 regarding existing requirements under the law or agency policies. The Directive includes a list of approved, online reprocessing antimicrobial systems for poultry. The regulatory compliance guidance clearly lists the key criteria and parameters expected to be controlled by the processor. The Directive also provides a complete list of safe and suitable ingredients summarized from the FCN and CFR references, plus labeling requirements and additional FSIS authorizations, if applicable (Table 5). The FSIS list supports the authorized uses from FDA, EPA, and USDA sources. Significantly, the FSIS list and CFR and FCN databases do not include corresponding efficacy/validation studies (log reduction) published in peer-reviewed scientific journals and private research accompanying pre-market review submissions.
Scientific challenge/validation studies alone are not Safe Harbors. They are the starting points to determine the feasibility for developing/commercializing antimicrobial interventions. For example, a recent study by Glass and Lim23 has shown that GRAS hydrogen peroxide (H2O2) at 100 ppm reduced L. monocytogenes by > 4-log in used commercial cheese brining solutions after 7 days of storage at 7.2 °C. The authors make no claim about regulatory approval, but point out that H2O2 is GRAS when used in milk intended for use in cheese-making (maximum level 500 ppm H2O2) and in several other foods listed in 21 CFR 184.1366. The 100-ppm level tested in the study is below the maximum level allowed in milk intended for cheese-making.
Per the regulation, H2O2 is affirmed as GRAS with specific limitation(s), and any use that is not in full compliance with each established limitation requires a food additive regulation. Unless there is a clear regulatory reference, a specific waver, or a "no objection" letter on file, there is no Safe Harbor.
Summary
Incidental contact sanitizers, antimicrobial processing aids, and antimicrobial food additives continue to play important roles in multi-hurdle, risk-based preventive control systems. Technological advancements contribute significantly to this clandestine warfare. Antimicrobial packaging incorporates minuscule agents into the material, continuously releasing a measured dose of protection against surface contamination. Even UV light devices,24 strategically employed in processing lines, effectively deactivate bacteria and viruses on surfaces and in liquids.
Many antimicrobial preventive control options are available that meet Safe Harbor criteria from a regulatory point of view. Some have been "grandfathered in," while others are more recent. The utilization of these processing aids is not without scrutiny. Ongoing research seeks novel and more targeted approaches, aiming for effective food protection without compromising taste or safety.
Processors and foodservice operators must keep in mind that food contact substances are highly regulated and add complexity to processes and training programs. They must also remember that food processing establishments and foodservice facilities, not regulatory agencies, are responsible for determining whether a non-food compound such as a cleaner, sanitizer, disinfectant, pesticide, antimicrobial treatment, or processing aid is safe, effective, and used within regulatory compliance guidelines. Food safety professionals must carefully consider Safe Harbor use ranges for all parameters (i.e., ppm, pH, temperature, spray time duration, spray pressure, coverage, contact time, and immersion dwell time) when planning, designing, and engineering the delivery system. The mixing and metering systems must be precise and are often automated, requiring sophisticated technical support during operation. Compliance with regulatory Safe Harbors is non-negotiable and must be verified at each applicable step in the supply chain from farm to fork.
Regulatory non-compliance associated with unapproved antimicrobial solutions or concentrations above regulatory thresholds that come into direct contact with food can result in adulteration, product loss, and product recalls, as well as criminal penalties. It is up to each company's leadership to determine if the preventive control processes in the supply chain are functional and align with the organization's food safety, quality, and social responsibility goals, so as not to harm a consumer or the company's brand.
References
- U.S. Food and Drug Administration (FDA). "Food Chemical Safety." Current as of March 27, 2024. https://www.fda.gov/food/food-ingredients-packaging/food-chemical-safety.
- U.S. Environmental Protection Agency (EPA). "Food Contact Sanitizing Solutions Model (FCSSM) For Estimating Indirect Dietary Exposure To Components of Sanitizing Solutions Used in Commercial Settings: User Guide." Version 1. April 2017. https://www.epa.gov/sites/default/files/2017-05/documents/fcssm-user-guide-version-1.pdf.
- U.S. Department of Agriculture Food Safety and Inspection Service (USDA-FSIS). "Nonfood Compounds." April 23, 2019. https://www.fsis.usda.gov/inspection/compliance-guidance/labeling/nonfood-compounds.
- USDA-FSIS. "Criteria Used by the Former Compounds and Packaging Branch for Evaluating Nonfood Compounds and Proprietary Substances." September 2013. https://www.fsis.usda.gov/guidelines/2013-0020.
- Powitz, R.W. "Non-EPA Registered Cleaners and Sanitizers for Use in Food Production Facilities and Retail Food Establishments." Food Safety Magazine. September 24, 3013. https://www.food-safety.com/articles/4251-non-epa-registered-cleaners-and-sanitizers-for-use-in-food-production-facilities-and-retail-food-establishments.
- FDA. "Inventory of Effective Food Contact Substance (FCS) Notifications." Page last updated February 29, 2014. https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=FCN.
- FDA. Code of Federal Regulations. "21 CFR 170.39: Threshold of regulation for substances used in food-contact articles." Title last amended April 11, 2024. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-170/subpart-B/section-170.39.
- FDA. Code of Federal Regulations. "21 CFR 101.100: Food; exemptions from labeling." Title last amended April 11, 2024. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-101/subpart-G/section-101.100.
- FDA. "Inventory of Effective Food Contact Substance (FCS) Notifications." Page last updated February 29, 2014. https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=FCN.
- USDA-FSIS. "Complete List of Safe and Suitable Ingredients Used in the Production of Meat, Poultry, and Egg Products." June 2023. https://www.fsis.usda.gov/sites/default/files/media_file/2021-09/7120.1_table_2.pdf.
- FDA. "Food Ingredient & Packaging Inventories." Current as of September 25, 2023. https://www.fda.gov/food/food-ingredients-packaging/food-ingredient-packaging-inventories.
- Ünlü, G. "Determining Infectious Doses of Foodborne Illness Agents." Food Technology Magazine. May 1, 2021. https://www.ift.org/news-and-publications/food-technology-magazine/issues/2021/may/columns/food-safety-and-quality-infectious-doses-of-foodborne-illness.
- Food Safety Magazine. "FDA Amends Food Contact Notification Regulations." March 21, 2024. https://www.food-safety.com/articles/9333-fda-amends-food-contact-notification-regulations.
- European Food Safety Authority (EFSA). "Microbiological hazards associated with the use of water in the post‐harvest handling and processing operations of fresh and frozen fruits, vegetables and herbs (ffFVHs) Part 1 (outbreak data analysis, literature review and stakeholder questionnaire)." EFSA Journal. November 3, 2023. https://www.efsa.europa.eu/en/efsajournal/pub/8332.
- Pangloli, P., Y.-C. Hung, L.R. Beuchat, C.H. King, and Z.-H. Zhao. "Reduction of Escherichia coli O157:H7 on Produce by Use of Electrolyzed Water under Simulated Food Service Operation Conditions." Journal of Food Protection 72, no. 9 (September 2009): 1854–1861. https://www.sciencedirect.com/science/article/pii/S0362028X22004835?via%3Dihub.
- Afari, G.W., Y.-C. Hung, C.H. King, and A. Hu. "Reduction of Escherichia coli O157:H7 and Salmonella Typhimurium DT 104 on fresh produce using an automated washer with near neutral electrolyzed (NEO) water and ultrasound." Food Control 63 (May 2016): 246–254. https://www.sciencedirect.com/science/article/abs/pii/S0956713515303091.
- Moorman, E. and H. King. "Is It Time for a 'Kill Step' for Pathogens on Produce at Retail?" Food Safety Magazine December 2016/January 2017. https://www.food-safety.com/articles/5104-is-it-time-for-a-e2809ckill-stepe2809d-for-pathogens-on-produce-at-retail.
- King, H. and W. Bedale. Hazard Analysis and Risk-Based Preventive Controls: Improving Food Safety in Human Food Manufacturing for Food Businesses. Elsevier (2018). https://doi.org/10.1016/C2015-0-06151-2.
- USDA Agricultural Marketing Service (AMS). "5026: The Use of Chlorine Materials in Organic Production and Handling." https://www.ams.usda.gov/rules-regulations/organic/handbook/5026 and USDA-AMS "Guidance: The Use of Chlorine Materials in Organic Production and Handling." March 20, 2024. https://www.ams.usda.gov/sites/default/files/media/5026.pdf.
- USDA-AMS. "Peracetic Acid Handling/Processing." March 3, 2016. https://www.ams.usda.gov/sites/default/files/media/Peracetic%20Acid%20TR%203_3_2016%20Handling%20final.pdf
- Conference for Food Protection. "Guide for Washing and Crisping Whole Raw Fruits and Vegetables at Food Establishments." August 20, 2021. http://www.foodprotect.org/media/guide/guide-for-washing-and-crisping-whole-raw-fruits-and-vegetables-pdf-document-10-25-21-002.pdf.
- FDA. "Recently Published GRAS Notices and FDA Letters." Current as of April 10, 2024. https://www.fda.gov/food/gras-notice-inventory/recently-published-gras-notices-and-fda-letters.
- Food Safety Magazine. "Study Examines Inactivation of Listeria by Hydrogen Peroxide in Commercial Cheese Brines." February 2, 2024. https://www.food-safety.com/articles/9176-study-examines-inactivation-of-listeria-by-hydrogen-peroxide-in-commercial-cheese-brines.
- EPA. "Compliance Advisory: EPA Regulations About UV Lights that Claim to Kill or Be Effective Against Viruses and Bacteria." October 2020. https://www.epa.gov/sites/default/files/2020-10/documents/uvlight-complianceadvisory.pdf.